Jack Colreavy
- Feb 14, 2023
- 4 min read
ABSI - CRISPR: the future of medicine
Every Tuesday afternoon we publish a collection of topics and give our expert opinion about the Equity Markets.
In February, CRISPR will notch up 10 years since its discovery in its ability to edit human genes. Generally in biotech, it takes up to 20 years for a discovery to be implemented into medicine. However, the revolutionary power that is CRISPR technology has seen enormous investment and fierce competition resulting in 2023 possibly being the year that first approved medicines hit the market. ABSI this week explains CRISPR and the potential treatments coming to market.
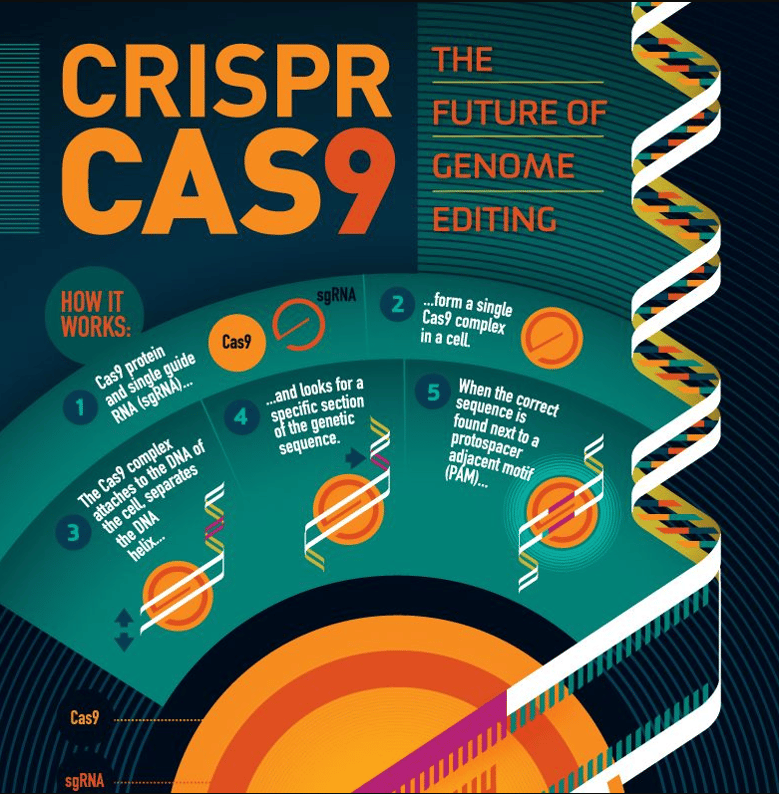
Clustered Regularly Interspaced Short Palindromic Repeats, better known as CRISPR, is the immune system for bacteria that cuts DNA damaged by a virus and replaces it with a new sequence. The main actors behind CRISPR are RNA, which can be programmed to target specific DNA sequences, and a protein called Cas9 (or other similar enzymes), which acts like a pair of molecular scissors to cut the DNA at the targeted location. Once the DNA is cut, the cell's natural repair mechanisms can be used to add or delete specific DNA sequences, effectively altering the organism's genetic code.
It is difficult to overstate the power of CRISPR, which has been repurposed by scientists to precisely target and modify specific DNA sequences within an organism's genome. Potential applications, including the treatment of genetic diseases, the improvement of crop yields, and the development of new therapies for a wide range of medical conditions.
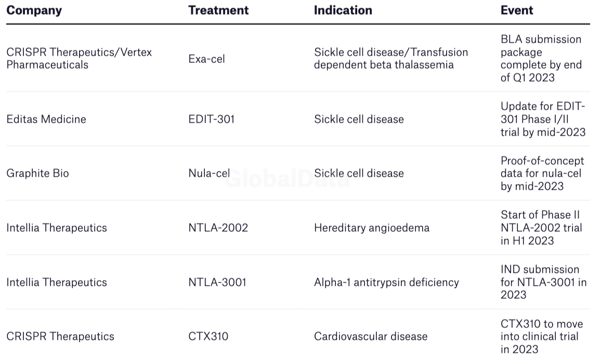
All eyes are on the CRISPR community in 2023, with the first approval decision due from the US FDA. CRISPR Therapeutics and Vertex Pharmaceuticals, should complete their Blood Licence Application (BLA) in Q1 2023 for their CRISPR-based treatment, named exa-cel, for sickle cell disease (SCD) and transfusion dependent beta thalassemia (TDT). The results from the clinical trials suggest that the treatment is a near-cure for the disease.
As another example, Caribou Biosciences, co-founded by one the Nobel prize winning CRISPR pioneers Jennifer Doudna, is working on a cancer treatment that edits a person’s immune cells to better target cancer cells. Although in early development, Caribou claims their Phase 1 trial resulted in all six non-Hodgkin's lymphoma patients being cancer-free after a single dose.
Select CRISPR-based catalyst updates in 2023
Source: Pharmaceutical Technology
While CRISPR has the potential to cure thousands of rare genetic diseases caused by just a single “misspelling” of the DNA code, it also has the real potential to create “super humans”. In theory, the tools could be used to increase stamina, muscle strength, intelligence, etc. Therefore it comes as no surprise that one the largest supporters of early CRISPR research was DARPA, the R&D arm of the US Defence Force.
Scientists and authorities are working hard on building out the ethical framework for CRISPR, but it doesn’t stop bad actors from taking the technology into their own hands. The biggest controversy in the field to-date was in November 2018 when a Chinese scientist, He Jiankui, reported that he had implanted gene-edited embryos in humans. The edits made to the embryos were for resistance to HIV and HIV-positive parents were recruited for the event. This eventually resulted in the birth of three CRISPR babies, two of which were twins, all HIV-free but it remains to be seen what other mutations may have resulted from the change to their DNA. Whilst claiming Chinese authorities were supportive of the experiment, Jiankui was ultimately sentenced to three years imprisonment as a result of the international condemnation.
CRISPR is a monumental step forward for humanity and has the ability to improve the lives of billions of humans. However, as with any new technology, it is important to proceed with caution and to consider the ethical and social implications of CRISPR before its widespread application. Nonetheless, I am very excited to see new CRISPR therapies begin to enter the market.
We offer value-rich content to our BPC community of subscribers. If you're interested in the stock market, you will enjoy our exclusive mailing lists focused on all aspects of the market.
To receive our exclusive E-Newsletter, subscribe to 'As Barclay Sees It' now.
Share Link